
However, these elements are reactive enough that they do not exist in their elemental forms in nature, but are present as compounds. alkali metal, any of the six chemical elements that make up Group 1 (Ia) of the periodic table namely, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Part of the reason why these elements react slowly is that these elements react with air to form a protective coating. The need to remove two electrons in order for the material to react means more energy is needed for electron removal. Question: Match each group of elements to the correct chemical reactivity trend observed based on valence electrons. However, it is possible to isolate elemental beryllium, magnesium, zinc, cadmium, mercury, aluminum, tin, and lead from their naturally occurring minerals and use them because they react very slowly with air. The Group 2 elements tend to be less reactive than their Group 1 counterparts. Beryllium does not react with water, magnesium reacts with steam. However, radium is a radioactive element and is generally under the category of radioisotopes in addition to being an alkaline earth metal, because it is not a stable element. The reactivity of the alkaline earth metal increases as you move down the column. Mixtures bubble and boil, fizz and hiss and may even smoke and burn. The reactions of alkali metals with water are pretty spectacular chemical reactions.

#Reactivity trend of alkali earth metals series
Radium (atomic number 88) has similar properties to barium and is also in the Group 2 category. Reactions of Alkali Metals Activity Series of Metals Introduction Elements are classified based on similarities, differences and trends in their properties, including their chemical reactions. What is the trend in reactivity for alkali metals Wiki User 14:10:20 Study now See answer (1) Best Answer Copy Metals Period - reactivity decreases as you go from left to. Do not do this experiment on the overhead. The two valence electrons are easily removed to form divalent cations.
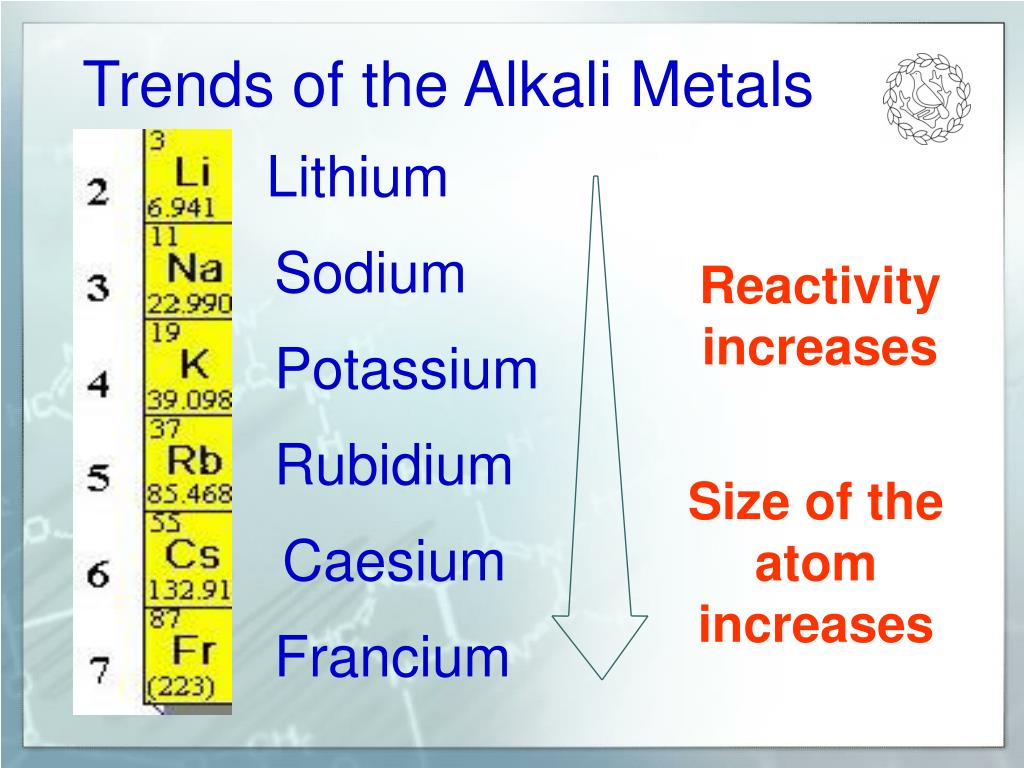
\) (Credit: Ingmar Runge Source: (opens in new window) License: Public Domain) The metals react vigorously with water such that even water in the air is enough to ignite sodium and potassium. They are highly reactive and are not found naturally in their elemental state.


 0 kommentar(er)
0 kommentar(er)
